We can't use an ensemble average directly for the entropy,
because it doesn't make sense to talk about the entropy of a microstate. But we can talk about
the entropy of the ensemble since the many copies can be in many different microstates. So we define
the entropy of the system as the entropy of the ensemble divided by the number of copies, ![]() , in the
ensemble:
, in the
ensemble:
![]() .
.
The ensemble has ![]() copies in the
copies in the ![]() th microstate, so the number of ways of arranging these is
th microstate, so the number of ways of arranging these is
So, using Stirling's approximation,
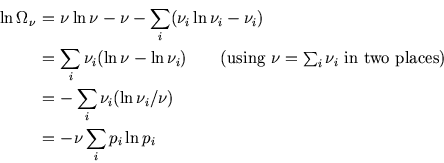
![$\mbox{\large\colorbox{yellow}{\rule[-3mm]{0mm}{10mm} \
$\displaystyle \left\langle S \right\rangle =-k_{\scriptscriptstyle B}\sum_i p_i\ln p_i$ }}$](img563.gif)
For a system in contact with a heat bath, ![]() is given by the Boltzmann distribution, so
is given by the Boltzmann distribution, so
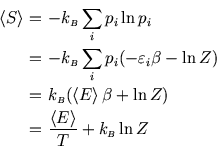
![$\mbox{\large\colorbox{yellow}{\rule[-3mm]{0mm}{10mm} \
$\displaystyle F=-k_{\scriptscriptstyle B}T \ln Z.$ }}$](img567.gif)