![$\mbox{\LARGE\colorbox{yellow}{\rule[-3mm]{0mm}{10mm} \
$\displaystyle F=-k_{\scriptscriptstyle B}T \ln Z$ }}$](img552.gif)
Here is the crucial equation which links the Helmholtz free energy and the partition function:
![$\mbox{\LARGE\colorbox{yellow}{\rule[-3mm]{0mm}{10mm} \
$\displaystyle F=-k_{\scriptscriptstyle B}T \ln Z$ }}$](img552.gif)
Since ![]() , from the
fundamental thermodynamic relation we obtain
, from the
fundamental thermodynamic relation we obtain
![]() . Thus
. Thus
(We first met these in the derivation of Maxwell's relations.)
For a magnetic system, we have
![]() instead
of the equation for P.
instead
of the equation for P.
Remember, ![]() and hence
and hence ![]() depend on
depend on ![]() (or
(or ![]() ) through the energies of the microstates. For instance
the energy levels of a particle in a box of side
) through the energies of the microstates. For instance
the energy levels of a particle in a box of side ![]() are proportional to
are proportional to
![]() .
.
These relations are reminiscent of those we met in the case of an isolated system, but there the entropy was the key; here it is the Helmholtz free energy. We can make the following comparison:
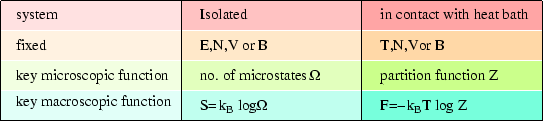
It should not surprise us to find that the Helmholtz free energy is the key to a system at fixed temperature (in contrast to the entropy for an isolated system) as that is what we found classically (see here.)
References