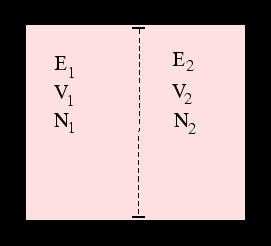

By considering two systems in contact with one another we can indeed deduce the existence of properties which determine whether they are in thermal, mechanical and diffusive equilibrium even if we knew no classical thermodynamics: these are the three partial derivatives of the entropy with respect to energy, volume and particle number.
Clearly these are related to the usual concepts of temperature, pressure and chemical potential. The correct
relations are the ones which reproduce the
fundamental thermodynamic relation
As an example, these ideas are applied to the paramagnet here. We can have our first go at the ideal gas now, too; see here.
References